Groth of Logarithmic Spirals
Human-produced materials are still far behind biomaterials. As bioscientists learn from DNAs and proteins, biominerals, such as teeth, bones, sea shells, and cell walls of phytoplanktons, also teach us a lot about something unexplored. They often show complicated but well-defined patterns, even characterized by hierarchical structures. For example, spicules of a sea urchin are known to give single crystal like spots on a X-ray diffraction pattern. Coccolith, a plankton abundant in the ocean, has scales on its surface, whose every part is again a single crystal. The living organisms grow those complicated morpholigies delivering high performances with violating usual prescribed polygonal shapes of single crystals surrounded by certain crystallographic faces like (111) face. Indeed, the riddle how they go beyond the well-studied mechanism had been left unsolved till a few decades ago. The recent progress in synthesizing and characterizing nanoscale materials is gradually lifting the lid of a treasure chest.
Several features of such biomineralization was being revealed. First, crystalization sometimes occurs through landing and micration of an ion on a crystal, but sometimes occurs through particles formation prior to landing and so on of them. The latter case sometimes allows further crystallographic transformation of particle-components, for instance, from amorphous to crystal accompanied by an increase of the density. Second, hybridization of two systems often gives results unwritten in textbooks. For example, crystalization of inorganic materials is considerably affected by the presence of organic coumpounds, which work as inhibitors by adsorbing selectively on a certain crystallographic face or as templates. In addition to the above two, there are more factors determining the resultant morphologies. It is good to read ref.[1] for further information.
On one hand, such biomineralization is still far away from something industrial or useful directly in our daily lives. On the other hand, nature likes it more than the textbook-style conventional crystalization, and moreover, makes meaningful use of it. The only problem is that we don't know why and how yet.
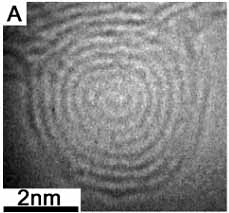
Among many interesting features of biomineralization, we are interested in how nature grows logarithmic spirals. The group leader previously observed a spiral growth of a carbon nanoparticle in a transmission electron microscope. When a nanoparticle of carbon black was converted to a nano-onion upon an intense electron irradiation, it appeared as an intermediate state (right). In this case, interlayer spacings is nearly constant. Such Archimedean spirals are familiar to materials scientists, like spiral dislocations of crystals. In contrast, logarithmic spirals are scarcely found in man-made products. Our technology allows no way to grow something like univalve shells.
On one hand, such biomineralization is still far away from something industrial or useful directly in our daily lives. On the other hand, nature likes it more than the textbook-style conventional crystalization, and moreover, makes meaningful use of it. The only problem is that we don't know why and how yet.
|
|
||
| |
Spherical carbon nanoparticle with a spiral structure[2].
|
|
Among many interesting features of biomineralization, we are interested in how nature grows logarithmic spirals. The group leader previously observed a spiral growth of a carbon nanoparticle in a transmission electron microscope. When a nanoparticle of carbon black was converted to a nano-onion upon an intense electron irradiation, it appeared as an intermediate state (right). In this case, interlayer spacings is nearly constant. Such Archimedean spirals are familiar to materials scientists, like spiral dislocations of crystals. In contrast, logarithmic spirals are scarcely found in man-made products. Our technology allows no way to grow something like univalve shells.
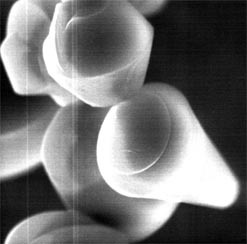
The figures below are micron-sized silica particles.  The presence of surfactants in the sol-gel reactions of tetraethoxy silane is known to give particles like the figures having unique morphologies. Sometimes spirals similar to univalve shells grow, and sometimes helices like coiling springs grow, depending on the growth conditions. Thus, collaboration between inorganic and organic systems is likely one of the keys to open the uncultivated lands of nature. Though our attempt has lately started, we are studying biomineralization from this viewpoint, especially how such logarithmic spirals grow.
The presence of surfactants in the sol-gel reactions of tetraethoxy silane is known to give particles like the figures having unique morphologies. Sometimes spirals similar to univalve shells grow, and sometimes helices like coiling springs grow, depending on the growth conditions. Thus, collaboration between inorganic and organic systems is likely one of the keys to open the uncultivated lands of nature. Though our attempt has lately started, we are studying biomineralization from this viewpoint, especially how such logarithmic spirals grow.
 The presence of surfactants in the sol-gel reactions of tetraethoxy silane is known to give particles like the figures having unique morphologies. Sometimes spirals similar to univalve shells grow, and sometimes helices like coiling springs grow, depending on the growth conditions. Thus, collaboration between inorganic and organic systems is likely one of the keys to open the uncultivated lands of nature. Though our attempt has lately started, we are studying biomineralization from this viewpoint, especially how such logarithmic spirals grow.
The presence of surfactants in the sol-gel reactions of tetraethoxy silane is known to give particles like the figures having unique morphologies. Sometimes spirals similar to univalve shells grow, and sometimes helices like coiling springs grow, depending on the growth conditions. Thus, collaboration between inorganic and organic systems is likely one of the keys to open the uncultivated lands of nature. Though our attempt has lately started, we are studying biomineralization from this viewpoint, especially how such logarithmic spirals grow.|
|
|
| Silica paricles grown in the presence of surfactants (grown in reference to [3]). |
|
[1] "Controlling mineral morphologies and structures in biological and synthetic systems" F.C. Meldrum and H. Cölfen, Chem. Rev. 108, 4332-4432 (2008).
[2] "Continuously growing spiral carbon nanoparticles as the intermediates in the formation. of fullerenes and nano-onions." M. Ozawa, H. Goto, M. Kusunoki, and E.Ōsawa, J. Phys. Chem. B 106, 7135-7138 (2002).
[3] "Shell mimetics" G. A. Ozin, H. Yang, I. Sokolov, and N. Coombs, Adv. Mater. 9, 662-667 (1997).