Self-Assmbly of Nanoparticles and Fabrication of Soft Materials
The remarkable development of science and technology we have experienced in the past 20th century looks getting even faster today. Meanwhile, its unsustainable character results in environmental issues. The way nature does is the way nature likes. To learn it is essential for us towards not only a highly functional but sustainable technology.
One of the propellants for scientific and technological development is based on the variety of materials being developed. Recently we are learning how to produce and use nanomaterials, and nanotechnology came out of it. Bottom up strategies are getting applicable to build devices up like creatures do for their own bodies. DNA assembles RNA, which assembles amino acids to produce proteins. Then, they come together to form hierarchical systems, including cells, enzymes, muscles and variety of organs. Biomaterials consequently realize the high-performance far beyond man-made stuffs in this way. Thus, to learn self-assembly of nanomaterials is one of the most important subjects.
Another issue what we should learn from biomaterials is to design soft materials (or soft matter). It is well known that 60% of our bodies is made of water, implying gels. They are tough, flexible with low friction, thermal, self-healing, etc.. Such soft materials are unfamiliar to our technology yet,while we handle hard materials rather well, compared with what nature does. As self-assembly and soft materials are correlated each other, we are studying how to assemble nanoparticles as well as to design soft materials using the techniques.
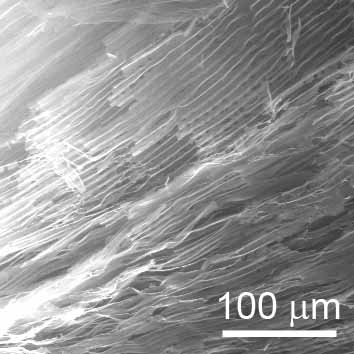
Among a number of approaches for self-assembly, one of the easiest ways is to utilize phase separation of two different materials. Directional freezing is a typical example of the approach. Upon freezing of a hydrocolloid, phase separation between ice and dispersed materials takes place. If the freezing process was performed directionally, the temperature gradient direct the growth of the ice grains. Followed by a freeze-drying process, a porous assembly having channels throughout the structure is obtained. The figure below left shows a SEM micrograph of a structure thus obtained from a hydrocolloid of detonation nanodiamond with surfactant. We found the technique also allows to align diamond nanoparticles on a substrate as shown in the figure below right. Even such a simple phenomenon like a phase separation upon freezing can sometimes utilize manipulate nanodots. It is exiting to find out simple principles to handle invisible tiny stuffs. At the same time, it it also interesting to study how nanowires or nanodiscs behave under the similar conditions.
|
|
|
|||||
| A SEM micrograph of a porous nanodiamond assembly. |
|
|||||
Going one step further, assembling nanomaterials in a foreign matrix is a challenging issue. As known in gelation of a gelatine aqueous solution, polymers form 3D networks in water, which hold water. If the polymers is substituted by carbon nanotubes, the gel would be much tougher. If water is substituted further by plastics or metals, they would be considerably reinforced. This idea can be combined, for instance, with the assembling technique using directional freezing. As the structure of the SEM micrograph above is similar to that of cardboard, it seems promising to use it for reinforcement. The anisotropic character is also expected to give the product anisotropic, such as in electronic, mechanical, and thermal properties.
At all events, our goal is to develop useful and interesting materials, like something soft but tough, functional, hopefully having hierarchical structures, and adaptable to the system on the earth. There's plenty of room to study the nature's strategies.